Research
Research in the Roy Lab employs an interdisciplinary approach that integrates immunology, virology, and molecular biology approaches to understand the mechanism of healthy immune response without generating cancer and autoimmunity. We also employ chemical biology approaches to develop therapeutic strategies to improve immune response to vaccination, cancer and autoimmunity.
B cell-T cell collaboration in controlling antibody response.
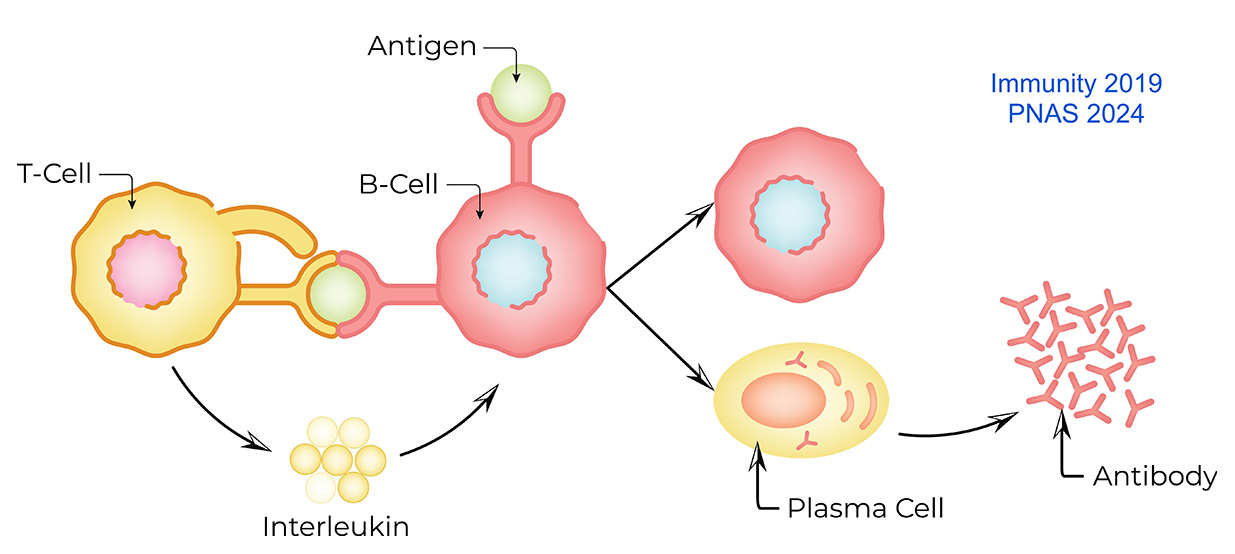
In humoral immunity, the first line of defense against reinfection is mediated by the pre-existing antibodies produced by plasma cells. High-quality antibodies prevent infections, whereas poor-quality antibodies can cause autoimmunity. Good-quality antibodies are generated by the tight regulation and collaboration of B cells and T cells in the germinal center. The quality of T cell-derived signals is a determinant of antibody quality. We are interested in understanding the role of T cell-derived signals in regulating B cell signaling and gene expression to produce high-quality antibodies while preventing the production of low-quality antibodies. We have engineered mouse models with the hope of transforming low-quality antibody-producing plasma cells into high-quality antibody-producing plasma cells.
Related Publications: PNAS 2018, Immunity 2019, PNAS 2024
Regulatory mechanism of memory B cell response
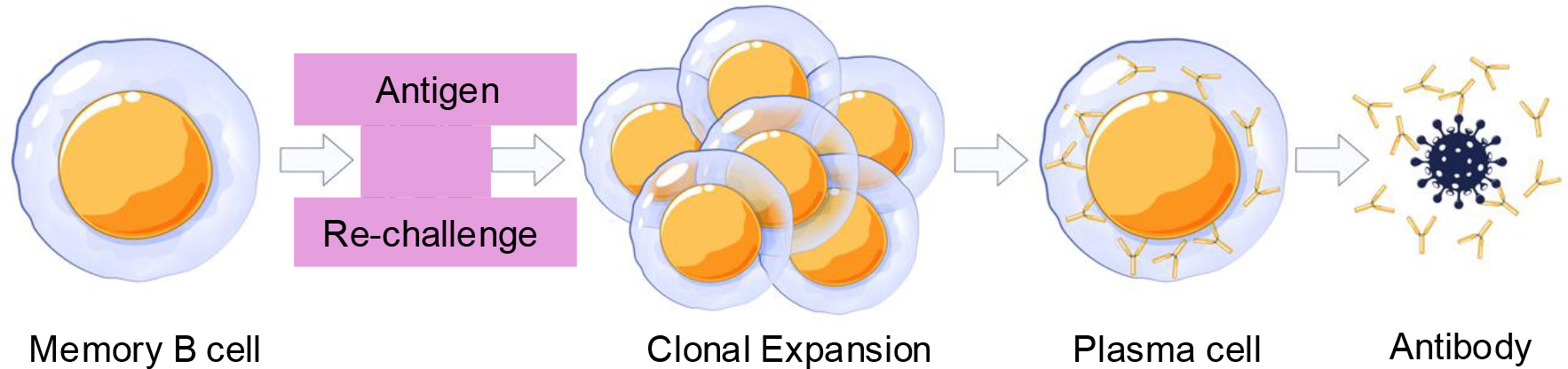
In humoral immunity, the second line of defense against reinfection is mediated by the memory B cells. Memory B cells, upon reencounter with the antigen, become activated to enter the proliferative program and differentiate into plasma cells. Memory B cells have different subsets, and each subset plays a distinct role in preventing infection. We are interested in understanding the signaling and gene regulatory network that controls the generation of memory B cell subsets and their clonal expansion and differentiation into plasma cells. We have engineered mouse models with the aim of enhancing memory responses to reinfection.
Effect of acute and chronic viral infection on cancer and autoimmunity
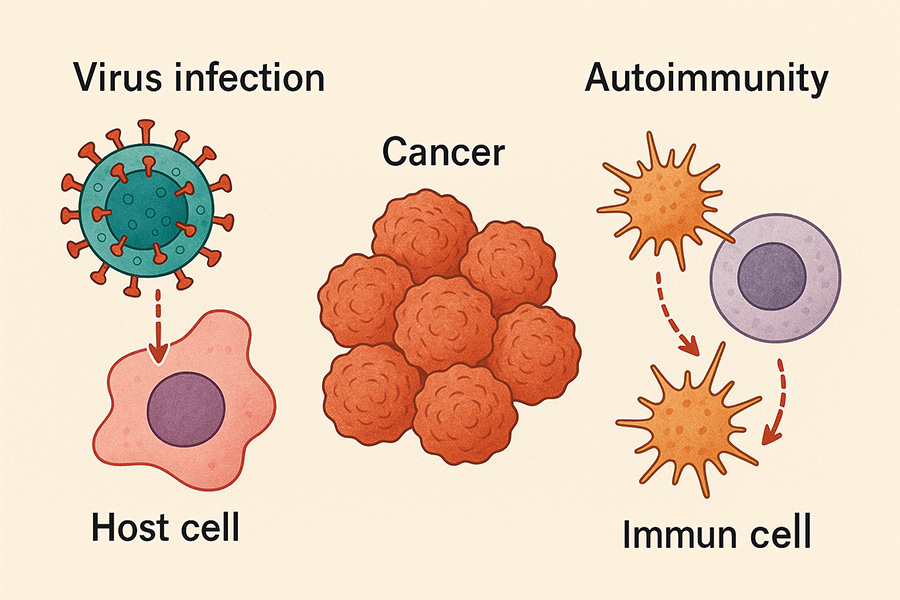
Acute and chronic infections impact our immune system differently and likely play different roles in cancer immunity and autoimmunity. Acute infections could prevent cancer development, while chronic infections could favor cancer development and autoimmunity. We are interested in understanding the B cell response to both acute and chronic infections, as well as their impact on the development of other diseases, including cancer and autoimmunity. The B cell response to acute and chronic infection depends on both B cell intrinsic and extrinsic factors. We are interested in understanding the role of both B cell intrinsic and extrinsic factors in the development of cancer and autoimmunity in chronic infections.
Peptide and protein-based immunotherapy for cancer and infection
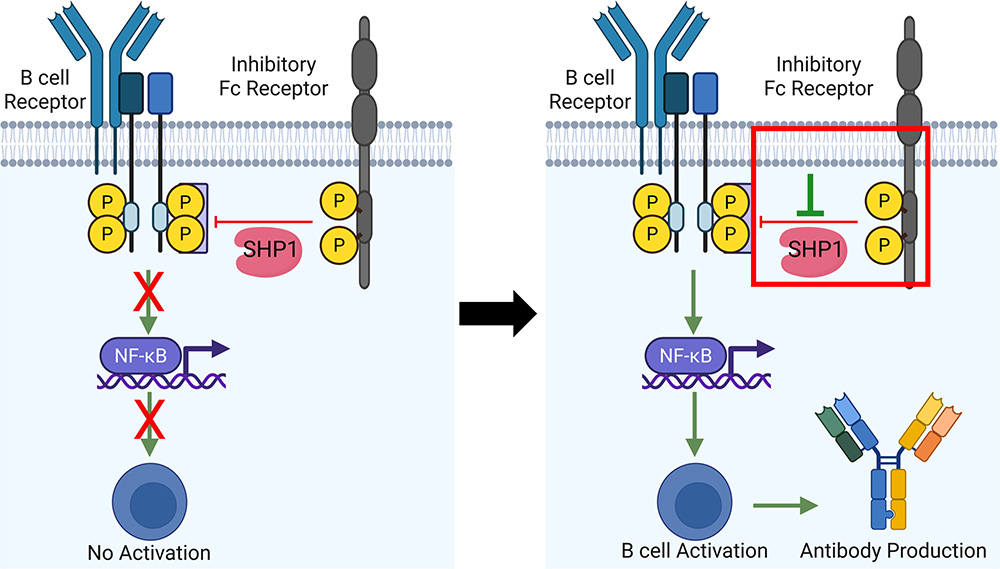
Peptide and protein-based therapeutic agents are advantageous over small molecules and large macromolecules due to their specificity, safety, low cost, and ease of handling. Thus, for the last few decades, chemical biologists have been utilizing peptides and proteins for various purposes, including vaccine candidates and immunotherapy for cancer. We have shown that a synthetic peptide-based inhibitor of Fc receptor enhances antibody production in a mouse model. Also, we have engineered a protein to design a synthetic transcription factor. Our research focuses on developing peptide and protein-based therapies with wide applications in various aspects of immunology, including vaccine and cancer immunotherapy.
Related Publications: Vaccine 2013, Chem Comm 2018, RSC Med Chem 2021